Abstract
Background: Allogeneic hematopoietic stem cell transplantation (HSCT) remains the only curative therapy in patients with primary or secondary myelofibrosis. Ruxolitinib, a JAK-inhibitor, has been approved in myelofibrosis patients and is effective to alleviate symptoms of the disease in a substantial proportion of patients. In a French phase 2 study, the use of ruxolitinib as a bridge to HSCT was followed by a high transplantation rate, with one third of patients presenting clinical improvement before HSCT (partial response or spleen size reduction) (BMT 2021, Robin et al). The aim of the current study is to compare post-transplantation outcomes between patients who received ruxolitinib before HSCT and those who did not.
Methods: This retrospective study includes patients with primary or secondary myelofibrosis registered in the SFGM-TC registry including patients from France, Belgium and Switzerland. Patients who received HSCT between July 2010 and December 2019 with available information regarding ruxolitinib treatment were included. The impact of ruxolitinib was analyzed by multivariable Cox regression and a propensity score.
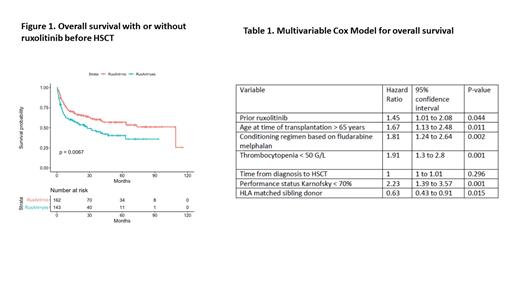
Results: 305 patients could be included, of whom 143 received ruxolitinib prior to HSCT (RuxoG). All patient characteristics are given in RuxoG followed by no ruxolitinib group (noRuxo). The diagnosis before HSCT was primary myelofibrosis (83% vs 75%), secondary myelofibrosis (13% vs 19%) or transformed into AML (4% vs 6%). Karnofsky score at transplant was < 70% in 9% and 11%. In RuxoG. median age was older (60.29 vs. 58.75, p=0.069) and delay from diagnosis longer (20.42 vs 13.26 months, p=0.040). RuxoG patients had less frequently anemia < 10 g/dl (84% vs 98%, p=0.027) or thrombocytopenia < 50 G/L (22% vs 32%, p=0.066) and presented more frequently splenomegaly or pre-graft splenectomy (83% vs 63%, p=0.0007). DIPSS score was well balanced in both groups, being high or intermediate-2 in 100 patients (79% vs 86%, p=0.23), from the 235 patients with available data. Myeloablative conditioning regimen was used in 16% vs 26% (p=0.049), and conditioning regimen consisted of on fludarabine-busulfan in 33% vs 61%, fludarabine-melphalan in 47% vs 22% or other in 20% vs 16% (p<0.0001). The donor was HLA-matched in 93% and 85%, with 31% vs 35% being matched sibling donor. Partial response or any other response before HSCT was reported in 41% (RuxoG) and 29% (noRuxo). Regarding post-transplant outcomes, cumulative incidence of grade 2-4 or 3-4 acute GVHD at day 100 was 46.5% and 32.2% in RuxoG and 40.4% and 20.7% in noRuxo group (p=0.11 and p=0.023, respectively). Chronic GVHD incidence at 24 months was 33.2% in RuxoG and 43.3% in noRuxo group (p=0.15) Overall survival was improved for noRuxo versus RuxoG, with 60 month OS at 40.3% and 57.0% (p=0.0067) (Figure 1). Causes of death were: HSCT related in 35 and 41 patients, related to GVHD in 65% and 43%, respectively. A multivariate Cox full model after selection and imputation showed that prior ruxolitinib significantly increased the risk of mortality (Table 1). Age, conditioning regimen, thrombocytopenia, performance status and type of donor were also prognostic. The propensity score confirmed that prior ruxolitinib was associated with higher risk of mortality (HR: 1.60, [95%CI:1.06 to 2.42]; p=0.024) including after adjustment for the delay from diagnosis to transplantation (HR: 1.59 [95%CI: 1.05 to 2.42], p=0.028). Of note, in patients responding to prior ruxolitinib, this deleterious effect was not observed.
Conclusion: Patients who received ruxolitinib before HSCT were at higher risk of acute GVHD and post-transplant mortality, but this was not observed in patients responding to ruxolitinib and it does not translate into higher risk of chronic GVHD. Main hypotheses to explain these finding could be: 1) specific disease, patient or transplant characteristics in RuxoG; 2) the longer delay to transplantation or 3) a specific effect of ruxolitinib. This study suggests that myelofibrosis patients responding to ruxolitinib should have the transplant without waiting disease progression.
Forcade: Novartis: Other: travel grant. Loschi: AbbVie: Ended employment in the past 24 months, Honoraria; CELGENE/BMS: Honoraria; Gilead: Ended employment in the past 24 months, Honoraria; Novartis: Ended employment in the past 24 months, Honoraria; Servier: Ended employment in the past 24 months, Honoraria; MSD: Honoraria. Duléry: Takeda Gilead Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Robin: NEOVII MEDAC NOVARTIS: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal